Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
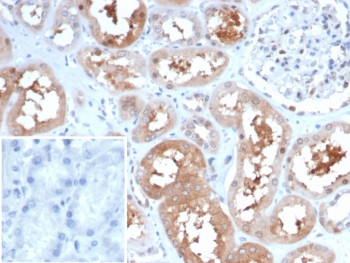
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| NSJ-V4368SAF-100UG | 100 µg | - | - |
3 - 10 business days* |
752.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
1 mg/ml in 1X PBS, BSA free, sodium azide free. The heat shock response was first described for... more
Product information "Anti-HSP90 alpha / HSP90AA1, clone HSP90AA1/7247"
1 mg/ml in 1X PBS, BSA free, sodium azide free. The heat shock response was first described for Drosophila salivary gland cells and morphologically consists of a change in their polytene chromosome puffing patterns that involves de novo synthesis of a few proteins. Similar heat shock proteins were later discovered in bacterial chicken and mammalian cells, and have been subsequently studied in other organisms. A series of proteins including HSP 90, HSP 70, HSP 20-30 and ubiquitin are induced by insults such as temperature shock, chemicals and other environmental stress. A major function of HSP 90 and other HSPs is to act as molecular chaperones. HSP 90 forms a complex with glucocorticoid receptor (GR), rendering the non ligand-bound receptor transcriptionally inactive. HSP 90 binds the GR as a heterocomplex composed of either HSP 56 or Cyclophilin D, forming an aporeceptor comiplex. HSP 90 also exists as a dimer with other proteins such as p60/sti1 and p23, forming an apo-receptor complex with estrogen and androgen receptors. Protein function: Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity which is essential for its chaperone activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function (PubMed:11274138, PubMed:15577939, PubMed:15937123, PubMed:27353360, PubMed:29127155, PubMed:12526792). Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself (PubMed:29127155). Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co- chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle (PubMed:27295069, PubMed:26991466). Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70 (PubMed:12526792). Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels (PubMed:25973397). In the first place, they alter the steady-state levels of certain transcription factors in response to various physiological cues(PubMed:25973397). Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment (PubMed:25973397). Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression (PubMed:25973397). Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes (PubMed:11276205). Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation (PubMed:24613385). Mediates the association of TOMM70 with IRF3 or TBK1 in mitochondrial outer membrane which promotes host antiviral response (PubMed:20628368, PubMed:25609812). [The UniProt Consortium]
| Keywords: | Anti-LAP-2, Anti-HSP86, Anti-HSP90A, Anti-HSP 86, Anti-Heat shock 86 kDa, Anti-LPS-associated protein 2, Anti-Heat shock protein HSP 90-alpha, Anti-Renal carcinoma antigen NY-REN-38, Anti-Lipopolysaccharide-associated protein 2, HSP90 alpha Antibody / HSP |
| Supplier: | NSJ Bioreagents |
| Supplier-Nr: | V4368SAF |
Properties
| Application: | IHC (paraffin) |
| Antibody Type: | Monoclonal |
| Clone: | HSP90AA1/7247 |
| Conjugate: | No |
| Host: | Mouse |
| Species reactivity: | human |
| Immunogen: | A recombinant partial protein sequence (within amino acids 500-700) from the human protein |
| Format: | Purified |
Database Information
| KEGG ID : | K04079 | Matching products |
| UniProt ID : | P07900 | Matching products |
| Gene ID | GeneID 3320 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | -20°C (International: -20°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed


