Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| ABE-42-1037-200 | 200 µg | - |
3 - 11 business days* |
656.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
HSP70 genes encode abundant heat-inducible 70-kDa HSPs (HSP70s). In most eukaryotes HSP70 genes... more
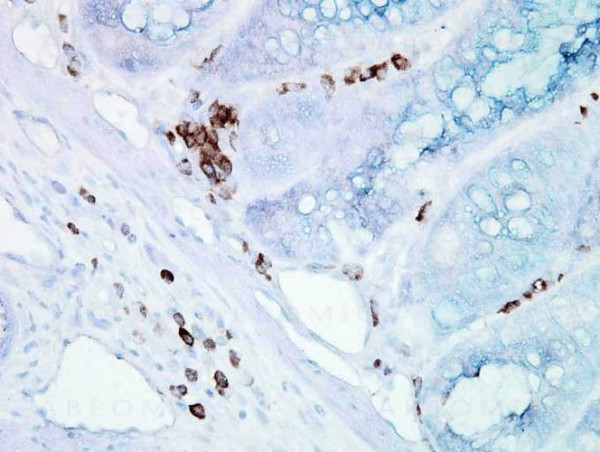
Product information "Anti-HSP70/HSC70 Monoclonal Antibody (Clone: BB70)"
HSP70 genes encode abundant heat-inducible 70-kDa HSPs (HSP70s). In most eukaryotes HSP70 genes exist as part of a multigene family. They are found in most cellular compartments of eukaryotes including nuclei, mitochondria, chloroplasts, the endoplasmic reticulum and the cytosol, as well as in bacteria. The genes show a high degree of conservation, having at least 50% identity. The N-terminal two thirds of HSP70s are more conserved than the C-terminal third. HSP70 binds ATP with high affinity and possesses a weak ATPase activity which can be stimulated by binding to unfolded proteins and synthetic peptides. When HSC70 (constitutively expressed) present in mammalian cells was truncated, ATP binding activity was found to reside in an N-terminal fragment of 44 kDa which lacked peptide binding capacity. Polypeptide binding ability therefore resided within the C-terminal half. The structure of this ATP binding domain displays multiple features of nucleotide binding proteins. All HSP70s, regardless of location, bind proteins, particularly unfolded ones. The molecular chaperones of the HSP70 family recognize and bind to nascent polypeptide chains as well as partially folded intermediates of proteins preventing their aggregation and misfolding. The binding of ATP triggers a critical conformational change leading to the release of the bound substrate protein. The universal ability of HSP70s to undergo cycles of binding to and release from hydrophobic stretches of partially unfolded proteins determines their role in a great variety of vital intracellular functions such as protein synthesis, protein folding and oligomerization and protein transport. Protein function: Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The affinity for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. It goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. [The UniProt Consortium]
| Keywords: | Anti-HSP70, Anti-Heat shock 70 kDa protein, Anti-HSP70/HSC70 Monoclonal Antibody (Clone: BB70) |
| Supplier: | Abeomics |
| Supplier-Nr: | 42-1037 |
Properties
| Application: | WB, IHC, IP |
| Antibody Type: | Monoclonal |
| Clone: | BB70 |
| Conjugate: | No |
| Host: | Mouse |
| Species reactivity: | human, mouse, rat, bovine, sheep, dog, guinea pig, swine, rabbit, chicken, Drosophila, yeast |
| Immunogen: | Chicken HSP70/HSP90 complex |
Database Information
| KEGG ID : | K03283 | Matching products |
| UniProt ID : | P08106 | Matching products |
| Gene ID | GeneID 423504 | Matching products |
Handling & Safety
| Storage: | +4°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed