Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| ABE-36-2509-20 | 20 µg | - |
3 - 11 business days* |
375.00€
|
|||
| ABE-36-2509-100 | 100 µg | - |
3 - 11 business days* |
853.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
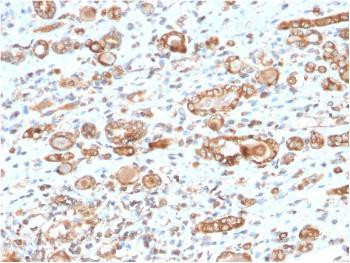
The heat shock proteins (HSPs) comprise a group of highly conserved, abundantly expressed... more
Product information "Anti-HSP60 (Heat Shock Protein 60) (Mitochondrial Marker) Monoclonal Antibody (Clone: CPTC-HSPD1-1)"
The heat shock proteins (HSPs) comprise a group of highly conserved, abundantly expressed proteins with diverse functions, including the assembly and sequestering of multiprotein complexes, transportation of nascent polypeptide chains across cellular membranes, and the regulation of protein folding. The mitochondrial and cytosolic localization of HSP60, combined with its binding and catalysis of folding of newly synthesized proteins destined for the mitochondrial matrix, classify this protein as a molecular chaperone. An additional role of HSP 60 is to act as a cell surface marker for T cell recognition, as well as being involved in a danger signal cascade immune response. HSP60 has been shown to influence apoptosis in tumor cells, and changes in its expression level may serve as a biomarker, as down-regulated HSP60 expression indicates a poor prognosis as well as a risk of tumor infiltration development, especially with regard to urothelial carcinomas. In ovarian cancer, decreased expression of HSP60 correlates with aggressive tumor types, while overexpression is correlated with a better patient prognosis. Protein function: Chaperonin implicated in mitochondrial protein import and macromolecular assembly. Together with Hsp10, facilitates the correct folding of imported proteins. May also prevent misfolding and promote the refolding and proper assembly of unfolded polypeptides generated under stress conditions in the mitochondrial matrix (PubMed:1346131, PubMed:11422376). The functional units of these chaperonins consist of heptameric rings of the large subunit Hsp60, which function as a back- to-back double ring. In a cyclic reaction, Hsp60 ring complexes bind one unfolded substrate protein per ring, followed by the binding of ATP and association with 2 heptameric rings of the co-chaperonin Hsp10. This leads to sequestration of the substrate protein in the inner cavity of Hsp60 where, for a certain period of time, it can fold undisturbed by other cell components. Synchronous hydrolysis of ATP in all Hsp60 subunits results in the dissociation of the chaperonin rings and the release of ADP and the folded substrate protein (Probable). [The UniProt Consortium]
| Keywords: | Anti-HSPD1, Anti-Hsp60, Anti-CPN60, Anti-HSP60, Anti-HSP-60, Anti-HuCHA60, Anti-Chaperonin 60, Anti-60 kDa chaperonin, Anti-Heat shock protein 60, Anti-P60 lymphocyte protein, Anti-Mitochondrial matrix protein P1, Anti-60 kDa heat shock protein, Anti-HSP6 |
| Supplier: | Abeomics |
| Supplier-Nr: | 36-2509 |
Properties
| Application: | FC, IF, WB, IHC |
| Antibody Type: | Monoclonal |
| Clone: | CPTC-HSPD1-1 |
| Conjugate: | No |
| Host: | Mouse |
| Species reactivity: | human |
| Immunogen: | Recombinant full-length human HSPD1 protein |
Database Information
| KEGG ID : | K04077 | Matching products |
| UniProt ID : | P10809 | Matching products |
| Gene ID | GeneID 3329 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed