Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| ABE-42-1197-100 | 100 µg | - |
3 - 11 business days* |
639.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
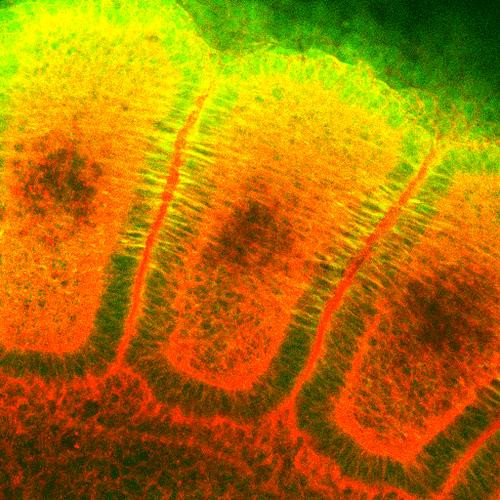
HSP25 is the mouse homologue of the human HSP27 protein, a member of the small heat shock protein... more
Product information "Anti-HSP27 Monoclonal Antibody (Clone: 8A7) - RPE"
HSP25 is the mouse homologue of the human HSP27 protein, a member of the small heat shock protein family comprised of a diverse group of proteins from ~15 to >30kDa(1). The basic structure of most sHSPs is a homologous and highly conserved amino acid sequence, with an alpha-crystallin-domain at the C-terminus and the WD/EPF domain at the less conserved N-terminus. This N-terminus is essential for the development of high molecular oligomers. HSP27-oligomers consist of stable dimers formed by as many as 8-40 HSP27 protein monomers. The oligomerization status is connected with the chaperone activity: aggregates of large oligomers have high chaperone activity, whereas dimers have no chaperone activity. HSP27 is localized to the cytoplasm of unstressed cells but can redistribute to the nucleus in response to stress, where it may function to stabilize DNA and/or the nuclear membrane. It can be rapidly phosphorylated in response to physiological stimuli relevant to the cell type examined. Thus, HSP27 has been suggested to be an important intermediate in second messenger-mediated signaling pathways. Other functions include chaperone activity (as mentioned above), thermo-tolerance in vivo, inhibition of apoptosis, and signal transduction. Specifically, in vitro, it acts as an ATP-independent chaperone by inhibiting protein aggregation and by stabilizing partially denatured proteins, which ensures refolding of the HSP70 complex. HSP27 is also involved in the apoptotic signaling pathway because it interferes with the activation of cytochrome c/Apaf-1/dATP complex, thereby inhibiting the activation of procaspase-9. It is also hypothesized that HSP27 may serve some role in cross-bridge formation between actin and myosin. And finally, HSP27 is also thought to be involved in the process of cell differentiation. The up-regulation of HSP27 correlates with the rate of phosphorylation and with an increase of large oligomers. It is possible that HSP27 may play a crucial role in termination of growth. Protein function: Small heat shock protein which functions as a molecular chaperone probably maintaining denatured proteins in a folding- competent state (PubMed:10383393, PubMed:20178975). Plays a role in stress resistance and actin organization (PubMed:19166925). Through its molecular chaperone activity may regulate numerous biological processes including the phosphorylation and the axonal transport of neurofilament proteins (PubMed:23728742). [The UniProt Consortium]
| Keywords: | Anti-SRP27, Anti-HspB1, Anti-HSP27, Anti-HSPB1, Anti-HSP 27, Anti-Heat shock 27 kDa protein, Anti-28 kDa heat shock protein, Anti-Heat shock protein beta-1, Anti-Stress-responsive protein 27, Anti-Estrogen-regulated 24 kDa protein, Anti-HSP27 Monoclonal A |
| Supplier: | Abeomics |
| Supplier-Nr: | 42-1197 |
Properties
| Application: | WB, IHC, ICC/IF, IP, FC |
| Antibody Type: | Monoclonal |
| Clone: | 8A7 |
| Conjugate: | Phycoerythrin |
| Host: | Mouse |
| Species reactivity: | human, mouse, rat, bovine, dog, guinea pig, sheep |
| Immunogen: | HSP27 peptide |
Database Information
| KEGG ID : | K04455 | Matching products |
| UniProt ID : | P04792 | Matching products |
| Gene ID | GeneID 3315 | Matching products |
Handling & Safety
| Storage: | +4°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed