Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| ABE-42-1353-200 | 200 µg | - |
3 - 11 business days* |
768.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
HSP70 genes encode abundant heat-inducible 70-kDa HSPs (HSP70s). In most eukaryotes HSP70 genes... more
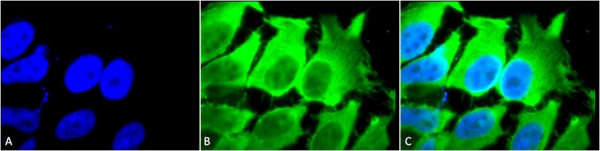
Product information "Anti-HSC70 (HSP73) Monoclonal Antibody (Clone: 1F2-H5) - APC"
HSP70 genes encode abundant heat-inducible 70-kDa HSPs (HSP70s). In most eukaryotes HSP70 genes exist as part of a multigene family. They are found in most cellular compartments of eukaryotes including nuclei, mitochondria, chloroplasts, the endoplasmic reticulum and the cytosol, as well as in bacteria. The genes show a high degree of conservation, having at least 50% identity. The N-terminal two thirds of HSP70s are more conserved than the C-terminal third. HSP70 binds ATP with high affinity and possesses a weak ATPase activity which can be stimulated by binding to unfolded proteins and synthetic peptides. When HSC70 (constitutively expressed) present in mammalian cells was truncated, ATP binding activity was found to reside in an N-terminal fragment of 44 kDa which lacked peptide binding capacity. Polypeptide binding ability therefore resided within the C-terminal half. The structure of this ATP binding domain displays multiple features of nucleotide binding proteins. When cells are subjected to metabolic stress (e.g., heat shock) a member of the HSP 70 family, HSP 70 (HSP72), is expressed, HSP 70 is highly related to HSC70 (>90% sequence identity). Constitutively expressed HSC70 rapidly forms a stable complex with the highly inducible HSP70 in cells following heat shock. The interaction of HSC70 with HSP 70 is regulated by ATP. These two heat shock proteins move together in the cell experiencing stress. Furthermore, research on HSC70 has implicates it with a role in facilitating the recovery of centrosomal structure and function after heat shock. Protein function: Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation (PubMed:21150129, PubMed:21148293, PubMed:24732912, PubMed:27916661, PubMed:23018488). This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones (PubMed:21150129, PubMed:21148293, PubMed:24732912, PubMed:27916661, PubMed:23018488). The co-chaperones have been shown to not only regulate different steps of the ATPase cycle of HSP70, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation (PubMed:21150129, PubMed:21148293, PubMed:24732912, PubMed:27916661, PubMed:23018488). The affinity of HSP70 for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. HSP70 goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The HSP70-associated co-chaperones are of three types: J- domain co-chaperones HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP- bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1 (PubMed:24318877, PubMed:27474739, PubMed:24121476, PubMed:26865365). Acts as a repressor of transcriptional activation. Inhibits the transcriptional coactivator activity of CITED1 on Smad-mediated transcription. Component of the PRP19-CDC5L complex that forms an integral part of the spliceosome and is required for activating pre-mRNA splicing. May have a scaffolding role in the spliceosome assembly as it contacts all other components of the core complex. Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes (PubMed:10722728, PubMed:11276205). Participates in the ER- associated degradation (ERAD) quality control pathway in conjunction with J domain-containing co-chaperones and the E3 ligase STUB1 (PubMed:23990462). [The UniProt Consortium]
| Keywords: | Anti-LAP-1, Anti-HSPA8, Anti-HSC70, Anti-LPS-associated protein 1, Anti-Heat shock 70 kDa protein 8, Anti-Heat shock cognate 71 kDa protein, Anti-Lipopolysaccharide-associated protein 1, Anti-HSC70 (HSP73) Monoclonal Antibody (Clone: 1F2-H5) - APC |
| Supplier: | Abeomics |
| Supplier-Nr: | 42-1353 |
Properties
| Application: | WB, IHC, ICC/IF, IP, ELISA |
| Antibody Type: | Monoclonal |
| Clone: | 1F2-H5 |
| Conjugate: | APC |
| Host: | Mouse |
| Species reactivity: | human, mouse, rat |
| Immunogen: | Full length human HSC70 |
Database Information
| KEGG ID : | K03283 | Matching products |
| UniProt ID : | P11142 | Matching products |
| Gene ID | GeneID 3312 | Matching products |
Handling & Safety
| Storage: | +4°C |
| Shipping: | +4°C (International: +4°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed