If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
Viewed
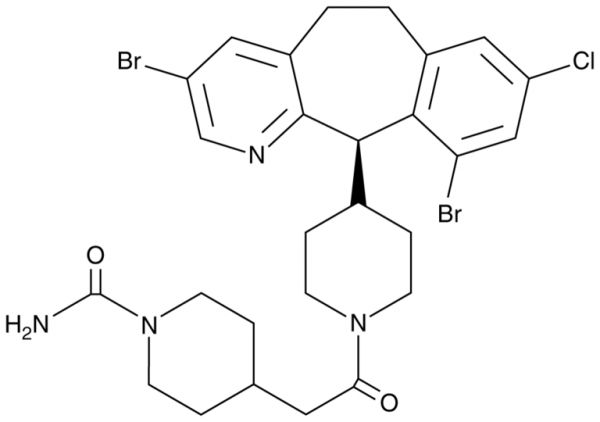
| Keywords: | Sarasar, SCH 66336, 4-[2-[4-[(11R)-3,10-dibromo-8-chloro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl]-1-piperidinyl]-2-oxoethyl]-1-piperidinecarboxamide |
| Supplier: | Cayman Chemical |
| Supplier-Nr: | 11746 |
Properties
| Application: | Farnesyl transferase inhibitor |
| MW: | 638.8 D |
| Formula: | C27H31Br2ClN4O2 |
| Purity: | >98% |
| Format: | Crystalline Solid |
Database Information
| CAS : | 193275-84-2| Matching products |
| KEGG ID : | K05954 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | +20°C (International: -20°C) |
| Signal Word: | Warning |
| GHS Hazard Pictograms: |
|
| H Phrases: | H361, H373 |
| P Phrases: | P201, P202, P260, P280, P308+313, P314, P405, P501 |
