Written by Emily Locke
When Beata Halassy’s breast cancer returned for the second time in 2020, she felt completely drained. Since the "triple-negative" tumor – a relatively rare and aggressive form of breast cancer – was first discovered four years earlier, she had already undergone two surgeries. She lost her left breast and endured an intensive course of chemotherapy. For Beata Halassy, one thing was clear: she did not want to go through this process again. So, she made a courageous decision. Halassy, a molecular biologist at the Center of Excellence for Virus Immunology and Vaccines at the University of Zagreb in Croatia, was conducting research on so-called oncolytic viruses (OVs) – a novel form of immunotherapy for cancer. Taking matters into her own hands, she decided to treat her breast cancer in a self-experiment using the viruses developed in her lab – with great success: Today, five years later, she remains tumor-free [1].
Although Halassy’s case has sparked a lively discussion in the scientific community regarding the ethical implications of self-experimentation in medicine, it also clearly demonstrates the enormous potential of oncolytic viruses in the fight against cancer [2]. One of the leading experts in this field in Germany is Prof. Dr. Dr. Guy Ungerechts. He leads the Clinical Cooperation Unit (CCU) for Virotherapy at the National Center for Tumor Diseases (NCT) in Heidelberg and has already conducted over ten early clinical trials with various oncolytic viruses [3]. Our student employee, Emily, had the unique opportunity to complete a three-month internship in his department, gaining valuable insights into the fascinating field of virotherapy research. In this blog post, she provides an overview of the mechanisms of action of oncolytic viruses and reports on her exciting research project.
These topics await you:
1) The Dual Nature of Viruses: From Disease to Cure
2) A Double Strike Against Cancer: How Oncolytic Viruses Fight Tumors
3) Measles Viruses for Tumor Vaccination: My Innovative Research Project
4) From the Laboratory to the Clinic: Translational Research at the NCT
Subscribe to the free Biomol Newsletter and never miss a Blog Article again!
The Dual Nature of Viruses: From Disease to Cure
Viruses have a bad reputation – especially since the COVID-19 pandemic, they are primarily seen by the general public as causes of infectious diseases. Additionally, some viruses (such as human papillomaviruses) can even trigger cancer and are responsible for around 12% of all cancer cases [4]. So, it might seem like one would want to avoid viruses altogether. However, this impression is misleading: viewing viruses solely as disease-causing agents overlooks their immense potential for innovative therapies and applications. For example, viruses have long been used as vectors in gene therapy to correct pathogenic mutations. Similarly, attenuated or inactivated viruses are used as vaccines, training the immune system and protecting against infectious diseases.
I had long been unaware that certain viruses can specifically target tumor cells. During my studies in Molecular Biotechnology, I never came across the term "oncolytic virotherapy" – likely because it is (still) a niche topic. However, since I have always been fascinated by viruses and their use in novel therapies, I decided to pursue a research internship in this field. Eventually, I found my place in the "Applied ViroTherapeutics" group within the CCU Virotherapy, led by Dr. Dr. Mathias Leber. The group focuses on developing an effective virotherapy based on the measles vaccine strain. Central to their work are the genetic modification of viral genomes and the exploration of combination therapies.
A Double Strike Against Cancer: How Oncolytic Viruses Fight Tumors
How did the unusual idea of using viruses to treat cancer even come about? The origins date back to the early 20th century, when clinical case reports first appeared describing patients who experienced a complete regression of their tumor after a viral infection. These observations led to the realization that certain viruses preferentially target cancer cells because these cells lack the natural defense mechanisms of healthy cells [5]. These discoveries laid the foundation for the field of oncolytic virotherapy. In this approach, viruses are genetically modified in the lab to specifically infect and destroy malignant cells. The direct "killing" of tumor cells through viral infection, replication, and spread is known as oncolysis and represents one of the main mechanisms of action of oncolytic viruses. Additionally, OVs act indirectly by triggering a local and systemic anti-tumor immune response. This occurs through the release of tumor antigens in the context of danger- and pathogen-associated molecular patterns (DAMPs and PAMPs), which create an immunogenic tumor microenvironment [6]. For this reason, oncolytic viruses are classified as cancer immunotherapies.

Figure 1: Overview of key strategies and advances in virotherapy (rearranged from [6]; Reprint under the Creative Commons Attribution 4.0 International License). (I) Development of novel oncolytic viruses by testing new strains/serotypes and generating fitness mutants. (II) Modification of viral capsids and pseudotyping to bypass antibody neutralization and improve targeting. (III) Integration of therapeutic transgenes such as suicide genes, cytokines, immune checkpoint antibodies, or tumor-associated antigens to enhance therapeutic efficacy. (IV) Targeted modification of entry and replication mechanisms, e.g., through capsid modifications or transcriptional control. (V) Combination therapies with radiotherapy, immunotherapies, epigenetics, or targeted therapies to optimize effectiveness. (VI) Clinical evaluation of oncolytic viruses in Germany, including parvoviruses, measles viruses, adenoviruses, vaccinia viruses, and other platforms.
In addition to the two main mechanisms of oncolytic virotherapy, research aims to further optimize the effectiveness and specificity of OVs through molecular modifications (Fig. 1). These include the identification and development of new viral strains or serotypes (Fig. 1.I), as well as the modification and pseudotyping of viral capsids to evade neutralization by antibodies (Fig. 1.II). Another central approach is to insert therapeutic transgenes into the viral genomes to develop so-called "armed" OVs (arming) (Fig. 1.III). Additionally, combination therapies with immunotherapy, chemotherapy, radiotherapy, and targeted therapies are being investigated (Fig. 1.V). Furthermore, scientists are working on specifically targeting oncolytic viruses to cancer cells. This can be achieved either at the cell entry level (entry targeting), for example, by fusing cancer-specific ligands with viral surface proteins, or during replication after cell entry (post-entry targeting) (Fig. 1.IV). The latter is achieved, for instance, by deleting viral genes or functions that are required for replication in healthy cells but not in cancer cells [6]. Many different virus platforms are currently being developed as OVs, including adenoviruses, herpes simplex viruses, measles viruses, parvoviruses, vaccinia viruses, and vesicular stomatitis viruses (Fig. 1.VI).
Measles Viruses for Tumor Vaccination: My Innovative Research Project
As mentioned earlier, the research group where I completed my internship focuses on the development of oncolytic measles viruses (oMeV). When most people hear the term "measles," they likely first think of the highly contagious, fever-inducing disease that primarily affects children. However, measles viruses also have another, surprisingly positive side: thanks to their natural oncotropism – the ability to preferentially infect cancer cells – they are considered promising candidates for virotherapy. In research, variants derived from the measles vaccine strain are used, which has already demonstrated an excellent safety profile during its long-term clinical use in both children and adults. Additionally, the negative-sense RNA virus, originating from the Paramyxoviridae family, offers versatile possibilities for genetic modification [7].
During my internship at the NCT, I had the great opportunity to contribute to the development of an oMeV-based therapeutic cancer vaccine for the treatment of solid tumors. The project, led by Dr. Dr. Mathias Leber and significantly advanced for over three years by medical doctoral student Juliane Hastedt, aims to release tumor antigens through oMeV-mediated oncolysis, thereby triggering a T-cell-based anti-tumor response to achieve long-term immunity (Fig. 2). Although the idea of developing therapeutic cancer vaccines has existed for several decades, successful implementation has been slow. One of the greatest challenges is the limited immunogenicity of earlier vaccine candidates, which is attributed to the various immunosuppressive strategies employed by cancer cells [8].

Figure 2: Dual mechanism of oncolytic measles viruses (translated from [7]; Reprint under the Creative Commons Attribution 4.0 International License). (1) Oncolysis: The oncolytic measles virus infects tumor cells, leads to their specific cell death, and releases tumor antigens (TAA/TSA), viral particles, and immunomodulatory cytokines. (2) Anti-tumor immune response: The released tumor antigens are taken up by dendritic cells, which trigger an immune response by activating T- and B-cells. This transforms a "cold" tumor into a "hot" tumor, contributing to an in situ vaccine effect and long-term tumor defense.
One of the rationales behind the project is that the oncolytic measles viruses we have developed are capable of overcoming immune suppression in the tumor microenvironment (TME). The oMeV-induced oncolysis results in the release of tumor-specific (TSA) and tumor-associated (TAA) antigens, as well as immunogenic PAMPs and DAMPs (Fig. 2.1). This process, known as immunogenic cell death (ICD), is intended to transform the previously immunologically "cold" TME into an inflammatory environment, thereby attracting immune cells to the site of infection. As a result of the recognition of TAA by lymphocytes, a tumor-specific immunological memory can develop, providing long-term protection against tumor recurrence (Fig. 2.2). This concept is often referred to as "in situ vaccination" [7].
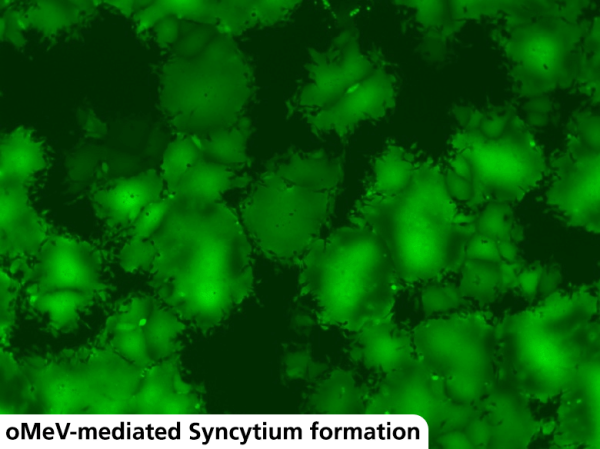
During my internship, I focused primarily on investigating and characterizing the toxicity and immunogenicity of the oMeVs developed by Juliane Hastedt. Since our measles virus encodes for the enhanced green fluorescent protein (eGFP), we are able to closely monitor tumor cell infection under the fluorescence microscope. One remarkable phenomenon observed is the formation of so-called syncytia – multinucleated cell units that result from the fusion of infected cells with neighboring cells (Fig. 3). The fusion of cell membranes is mediated by the interaction of the viral H and F glycoproteins with cellular receptors such as CD46 and CD150. The virus specifically exploits syncytium formation to spread efficiently from cell to cell without traversing the extracellular environment [9].

Figure 3: oMeV-infected Vero cells under the fluorescence microscope. Infection with the measles virus leads to the formation of multinucleated giant cells, known as syncytia.
From the Laboratory to the Clinic: Translational Research at the NCT
What excites me most about the research work at the CCU Virotherapy is that all projects are highly translational. My internship not only provided fascinating insights into the development of oncolytic viruses, but also showed me how closely laboratory work and clinical application are interconnected (Fig. 4). Many of the oncolytic viruses being studied by researchers at the NCT are candidates for clinical trials. The CCU is not only focused on developing innovative strategies for effective OVs, but also establishes GMP-compliant manufacturing processes for virotherapeutics and conducts clinical virotherapy studies [3]. The NCT offers the ideal environment for this: as a leading oncology center, it combines patient care and patient-related research under one roof. This allows newly developed therapies to be directly tested in clinical trials on-site.

Figure 4: Insights into my daily lab routine during the internship. Left: Work in the S2 cell culture room. Right: Collaborative analysis of an experiment with my supervisor, Juliane Hastedt.
The primary goal of the CCU Virotherapy is, of course, to bring the most effective oncolytic immunotherapies into clinical practice. So far, the list of approved oncolytic virotherapies is still limited: In 2015, Imlygic® (Talimogene Laherparepvec) became the first OV to be approved in both the USA and Europe. This genetically modified herpes simplex virus, which expresses GM-CSF (granulocyte-macrophage colony-stimulating factor), is used to treat advanced malignant melanoma [10]. Another genetically modified herpes simplex virus, Delytact® (Teserpaturev), received conditional and time-limited marketing approval in Japan in 2021 for the treatment of malignant gliomas [11]. At the CCU Virotherapy, there is hope that soon, more OVs will be added to the list of approved therapies. The prospects are promising: By the end of 2023, twelve clinical trials involving eight different OVs had already been conducted or initiated at the NCT [7]. And who knows – perhaps the oncolytic measles virus from my research project will one day also make the leap into clinical practice!
Sources
[1] https://www.zeit.de/gesundheit/2024-11/beata-halassy-selbstversuch-brustkrebs-medizin, 22.01.2025.
[2] Forčić D, Mršić K, Perić-Balja M, Kurtović T, Ramić S, Silovski T, Pedišić I, Milas I, Halassy B. An Unconventional Case Study of Neoadjuvant Oncolytic Virotherapy for Recurrent Breast Cancer. Vaccines (Basel). 2024 Aug 23;12(9):958.
[3] https://www.nct-heidelberg.de/das-nct/kernbereiche/medizinische-onkologie/forschung/virotherapie.html, 22.01.2025.
[4] Ameya G, Birri DJ. The molecular mechanisms of virus-induced human cancers. Microb Pathog. 2023 Oct;183:106292.
[5] Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007 Apr;15(4):651-9.
[6] Nettelbeck, D.M.; Leber, M.F.; Altomonte, J.; Angelova, A.; Beil, J.; Berchtold, S.; Delic, M.; Eberle, J.; Ehrhardt, A.; Engeland, C.E.; et al. Virotherapy in Germany—Recent Activities in Virus Engineering, Preclinical Development, and Clinical Studies. Viruses 2021, 13, 1420.
[7] Dittus, K., Eilert, A.E., Szczeponik, M.G. et al. Onkolytische Viren zur Behandlung von Krebserkrankungen. Biospektrum 29, 585–588 (2023).
[8] https://www.nct-heidelberg.de/das-nct/kernbereiche/medizinische-onkologie/forschung/virotherapie/discover.html, 22.01.2025.
[9] Burton C, Bartee E. Syncytia Formation in Oncolytic Virotherapy. Mol Ther Oncolytics. 2019 Oct 1;15:131-139.
[10] https://www.ema.europa.eu/en/medicines/human/EPAR/imlygic, 22.01.2025.
[11] Frampton JE. Teserpaturev/G47Δ: First Approval. BioDrugs. 2022 Sep;36(5):667-672.
Preview Image: Emily Locke, own recording (17.01.2025)