Fluorescent indicators that show spectral responses upon binding calcium have enabled researchers to investigate changes in intracellular free calcium concentrations by using fluorescence microscopy, flow cytometry, fluorescence spectroscopy and fluorescence microplate readers. When selecting a fluorescent calcium indicator, there are quite a few factors to consider, these include:
1. Indicator Form (AM Ester, Salt or Dextran Conjugate)
Form will determine which cell-loading technique is most appropriate. AM ester conjugated calcium indicators are cell-permeable allowing them to passively diffuse across intact plasma membranes. Salt and dextran-conjugate indicators are impermeable and require disruptive loading into cells by microinjection, patch pipette, electroporation or pinocytic loading agent, or used for extracellur assays.
2. Mode (Single-Wavelength & Ratiometric Indicators)
Fluorescence ion indicators fall under two categories: single-wavelength or ratiometric indicators. For qualitative analysis, single-wavelength indicators are recommended. Their large dynamic range and varying dissociation constants make them sensitive at detecting modest and transient calcium changes. Upon calcium chelation, single-wavelength indicators can increase their fluorescence intensity greater than 100-fold. Ratiometric, or dual-wavelength, indicators are recommended for the quantitative measurements of ionic concentrations. Raiometric indicators possess a unique spectral property which permits a shift in their optimum absorption or emission wavelength intensities when binding to free calcium. Ratios derived from this photometric data, can accurately determine intracellular calcium concentrations by reducing the adverse effects of uneven dye loading, dye leakage, photobleaching and changes in cell volume.
3. Spectral Properties
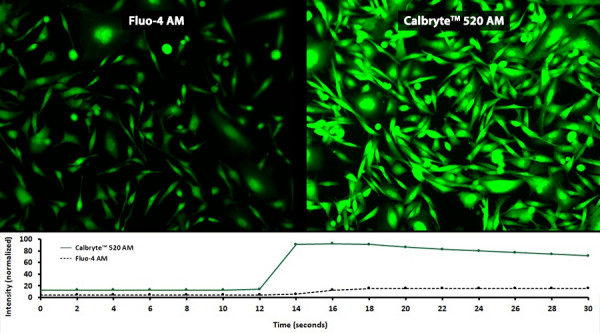
Indicator excitation and emission wavelengths are largely dependent upon the type of instrumentation, excitation source and sample to be used. For example, consider long-wavelength indicators, such as Calbryte™ 630 AM, for deep tissue calcium imaging or when multiplexing with a green calcium indicator.
4. Dissociation Constant (Kd)
The dissociation constant describes how tightly an indicator can bind calcium ions. Its value corresponds to the concentration at which half of the indicators are bound to calcium at equilibrium. Select an indicator that has an appropriate Kd compatible with the calcium concentration range of interest. The Kd values of calcium indicators are dependent upon many factors including pH, temperature, ionic strength, viscosity, protein binding, the presence of Mg2+ and other ions. Kd values for intracellular indicators are typically higher than the corresponding values measured in cell-free solutions.
What Is an AM Ester?
The acronym 'AM' represents an acetoxymethyl group, and AM esters are primarily used for the modification of negatively charged carboxylate compounds. By their nature, negatively charged compounds are cell-impermeant and require invasive loading techniques. AM ester modification masks the negatively charged carboxylic groups and produces uncharged hydrophobic indicators or chelators. The resulting cell-permeable AM derivatives can be invasively loaded into live cells for analysis. Once inside the cell, nonspecific intracellular esterases, hydrolyze (cleave) the AM groups. Removal of the AM groups, results in strongly fluorescent indicators or chelators that are well-retained within the cytoplasm.