If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
Viewed
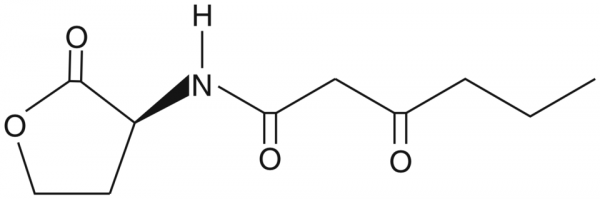
| Keywords: | 3-O-C6-(L)-HSL, 3-oxo-N-[(3S)-tetrahydro-2-oxo-3-furanyl]-hexanamide |
| Supplier: | Cayman Chemical |
| Supplier-Nr: | 10011207 |
Properties
| Application: | Antibiotic |
| MW: | 213.2 D |
| Formula: | C10H15NO4 |
| Purity: | >95% |
| Format: | Crystalline Solid |
Database Information
| CAS : | 143537-62-6| Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | +20°C (International: -20°C) |
