Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
Thapsigargin is an inhibitor of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA, IC50 = ~30... more
Product information "Thapsigargin"
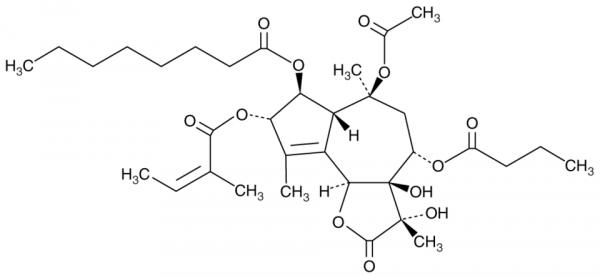
Thapsigargin is an inhibitor of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA, IC50 = ~30 nM for the rat liver microsomal enzyme), an ER stress inducer, and a sesquiterpene lactone that has been found in Thapsia. It increases intracellular calcium levels in isolated rat hepatocytes (EC50 = ~80 nM) and protein levels of the ER stress markers DNA damage-inducible transcript 3 (DDIT3), also known as CHOP, and glucose-regulated protein 78 kDa (GRP78), as well as phosphorylation of protein kinase R-like ER kinase (PERK) and eukaryotic translation initiation factor 2alpha subunit (eIF2alpha) in SH-SY5Y cells when used at a concentration of 1 µM. Acute exposure of thapsigargin (2 µg/ml for 1 h) to primary mouse bone marrow-derived macrophages (BMDMs) protects against TNF- and zVAD-induced necroptosis and prolonged exposure of thapsigargin (2-16 µM for 48 h) induces apoptosis in SW-13 adrenocortical carcinoma cells. Thapsigargin (3 µM) induces autophagosome accumulation in mouse embryonic fibroblasts (MEFs). It reduces viral titers in Vero E6 cells co-infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza A strain H1N1 when used at a concentration of 0.5 µM. Thapsigargin (1 mg/kg) reduces tumor volume in an SW-13 mouse xenograft model.Formal Name: (3S,3aS,4R,6R,7S,8R)-6-acetoxy-4-(butyryloxy)-3,3a-dihydroxy-3,6,9-trimethyl-8-(((Z)-2-methylbut-2-enoyl)oxy)-2-oxo-2,3,3a,4,5,6,6a,7,8,9b-decahydro-1H-cyclopenta[e]azulen-7-yl octanoate. CAS Number: 67526-95-8. Molecular Formula: C34H50O12. Formula Weight: 650.8. Purity: >97%. Formulation: (Request formulation change), A crystalline solid. Solubility: DMF: 30 mg/ml, DMSO: 30 mg/ml, Ethanol: 30 mg/ml, Ethanol:PBS (pH 7.2)(1:2): 0.3 mg/ml. SMILES: C[C@]1(OC(C)=O)C[C@H](OC(CCC)=O)[C@@]2(O)[C@](OC([C@@]2(C)O)=O)([H])C3=C(C)[C@H](OC(/C(C)=C\C)=O)[C@@H](OC(CCCCCCC)=O)C13. InChi Code: InChI=1S/C34H50O12/c1-9-12-13-14-15-17-24(37)43-28-26-25(20(5)27(28)44-30(38)19(4)11-3)29-34(41,33(8,40)31(39)45-29)22(42-23(36)16-10-2)18-32(26,7)46-21(6)35/h11,22,26-29,40-41H,9-10,12-18H2,1-8H3/b19-11-/t22-,26?,27-,28-,29-,32-,33+,34+/m0/s1. InChi Key: IXFPJGBNCFXKPI-JUVUYJTQSA-N. Origin: Plant/Thapsia garganica.
| Keywords: | (3S,3aS,4R,6R,7S,8R)-6-acetoxy-4-(butyryloxy)-3,3a-dihydroxy-3,6,9-trimethyl-8-(((Z)-2-methylbut-2-enoyl)oxy)-2-oxo-2,3,3a,4,5,6,6a,7,8,9b-decahydro-1H-cyclopenta[e]azulen-7-yl octanoate |
| Supplier: | Cayman Chemical |
| Supplier-Nr: | 10522 |
Properties
| Application: | SERCA inhibitor |
| MW: | 650.8 D |
| Formula: | C34H50O12 |
| Purity: | >97% |
| Format: | Crystalline Solid |
Database Information
| CAS : | 67526-95-8| Matching products |
| KEGG ID : | K05853 | Matching products |
Handling & Safety
| Storage: | -20°C |
| Shipping: | +20°C (International: -20°C) |
| Signal Word: | Danger |
| GHS Hazard Pictograms: |
|
| H Phrases: | H315, H319, H334, H335 |
| P Phrases: | P261, P264, P271, P280, P284, P312, P321, P302+P352, P304+P340, P304+P341, P305+P351+P338, P332+P313, P337+P313, P342+P311, P362+P364, P405, P403+P233, P501 |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed




