Cookie preferences
This website uses cookies, which are necessary for the technical operation of the website and are always set. Other cookies, which increase the comfort when using this website, are used for direct advertising or to facilitate interaction with other websites and social networks, are only set with your consent.
Configuration
Technically required
These cookies are necessary for the basic functions of the shop.
"Allow all cookies" cookie
"Decline all cookies" cookie
CSRF token
Cookie preferences
Currency change
Customer-specific caching
FACT-Finder tracking
Individual prices
Selected shop
Session
Comfort functions
These cookies are used to make the shopping experience even more appealing, for example for the recognition of the visitor.
Note
Show the facebook fanpage in the right blod sidebar
Statistics & Tracking
Affiliate program
Conversion and usertracking via Google Tag Manager
Track device being used
| Item number | Size | Datasheet | Manual | SDS | Delivery time | Quantity | Price |
|---|---|---|---|---|---|---|---|
| Cay10944-100 | 100 µg | - |
6 - 10 business days* |
500.00€
|
If you have any questions, please use our Contact Form.
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
You can also order by e-mail: info@biomol.com
Larger quantity required? Request bulk
WD repeats are motifs of approximately 40 amino acids typically terminating in conserved... more
Product information "WDR5 (human, recombinant)"
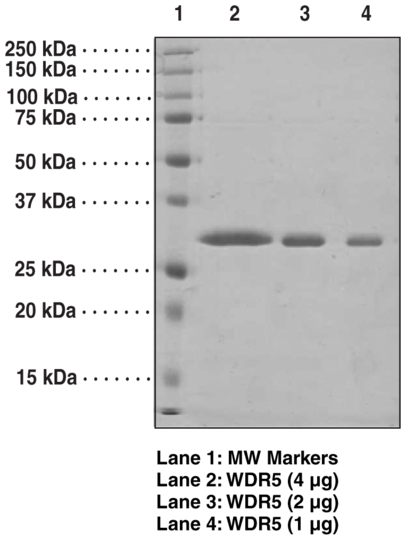
WD repeats are motifs of approximately 40 amino acids typically terminating in conserved tryptophan and aspartate residues. Several WD40 repeats combine to form the structural WD domain. Such an arrangement facilitates protein-protein interactions allowing the formation of multiprotein complexes. Human WDR5 contains seven WD 40 repeats and was originally identified to accelerate osteoblast differentiation. WDR5 has been demonstrated to bind histone H3 by recognizing the first three amino acids of the N-terminal tail. Binding of WDR5 to a conserved arginine-containing motif in MLL-1, the so-called WDR5 interaction ("Win") motif, promotes the assembly and activity of the MLL core complex. Additional interactions have been demonstrated with other components of the human MLL core protein complex, which includes ASH2L and RbBP5. MLL1-5 protein complexes catalyze the di- and trimethylation of histone H3 at lysine 4 (H3K4me2/me3), leading to the maintenance of global H3K4 trimethylation.Synonyms: BIG3, BMP2-induced 3-kb Gene Protein, SWD3, Set1c WD40 repeat protein, homolog, WD-Repeat Protein 5. Purity: >95% estimated by SDS-PAGE. Source: recombinant protein expressed in E. coli. Amino Acids: 2-334 (full length). MW: 34.4 kDa. Formulation: (Request formulation change), 50 mM Tris, pH 8.0, with 150 mM sodium chloride and 20% glycerol. License: SUMOpro tag was used under non-exclusive license from LifeSensors, Inc..
| Keywords: | BIG3, BMP2-induced 3-kb Gene Protein, SWD3, Set1c WD40 repeat protein, homolog, WD-Repeat Protein 5 |
| Supplier: | Cayman Chemical |
| Supplier-Nr: | 10944 |
Properties
| Application: | Enzyme activity |
| Conjugate: | No |
| Host: | E.coli |
| MW: | 34,4 kD |
| Purity: | >95% estimated by SDS-PAGE |
| Format: | Solution |
Database Information
| KEGG ID : | K14963 | Matching products |
| UniProt ID : | P61964 | Matching products |
Handling & Safety
| Storage: | -80°C |
| Shipping: | -80°C (International: -80°C) |
Caution
Our products are for laboratory research use only: Not for administration to humans!
Our products are for laboratory research use only: Not for administration to humans!
Information about the product reference will follow.
more
You will get a certificate here
Viewed






