Cookie-Einstellungen
Diese Website benutzt Cookies, die für den technischen Betrieb der Website erforderlich sind und stets gesetzt werden. Andere Cookies, die den Komfort bei Benutzung dieser Website erhöhen, der Direktwerbung dienen oder die Interaktion mit anderen Websites und sozialen Netzwerken vereinfachen sollen, werden nur mit Ihrer Zustimmung gesetzt.
Konfiguration
Technisch erforderlich
Diese Cookies sind für die Grundfunktionen des Shops notwendig.
"Alle Cookies ablehnen" Cookie
"Alle Cookies annehmen" Cookie
Ausgewählter Shop
CSRF-Token
Cookie-Einstellungen
FACT-Finder Tracking
Individuelle Preise
Kundenspezifisches Caching
Session
Währungswechsel
Komfortfunktionen
Diese Cookies werden genutzt um das Einkaufserlebnis noch ansprechender zu gestalten, beispielsweise für die Wiedererkennung des Besuchers.
Facebook-Seite in der rechten Blog - Sidebar anzeigen
Merkzettel
Statistik & Tracking
Endgeräteerkennung
Kauf- und Surfverhalten mit Google Tag Manager
Partnerprogramm
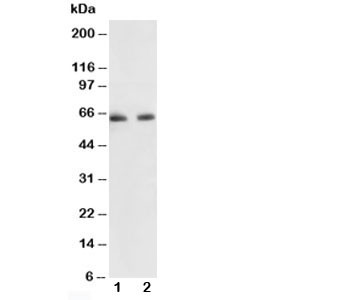
| Artikelnummer | Größe | Datenblatt | Manual | SDB | Lieferzeit | Menge | Preis |
|---|---|---|---|---|---|---|---|
| NSJ-R30293 | 100 µg | - | - |
3 - 10 Werktage* |
755,00 €
|
Bei Fragen nutzen Sie gerne unser Kontaktformular.
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
Bestellen Sie auch per E-Mail: info@biomol.com
Größere Menge gewünscht? Bulk-Anfrage
0.5mg/ml if reconstituted with 0.2ml sterile DI water. The p65 (RELA) heterodimer is the most... mehr
Produktinformationen "Anti-p65 NF-kB"
0.5mg/ml if reconstituted with 0.2ml sterile DI water. The p65 (RELA) heterodimer is the most abundant form of NF-KB. This gene is located on 11q13, which consists of 10 exons and spans about 8.1 kb of DNA. In rat sciatic nerves, the expression of the activated p65 subunit of NFKB was high in the nuclei of premyelinating Schwann cells and then progressively declined until it was nearly absent in adults. The transcriptional activity of NF-kappa-B is stimulated upon phosphorylation of its p65 subunit on serine-276 by protein kinase A(PKA). The transcriptional coactivator CBP/p300 associates with NF-kappa-B through 2 sites, an N-terminal domain that interacts with the C-terminal region of the unphosphorylated protein, and a second domain that only interacts with p65 phosphorylated on serine-276. Protein function: NF-kappa-B is a pleiotropic transcription factor present in almost all cell types and is the endpoint of a series of signal transduction events that are initiated by a vast array of stimuli related to many biological processes such as inflammation, immunity, differentiation, cell growth, tumorigenesis and apoptosis. NF-kappa-B is a homo- or heterodimeric complex formed by the Rel-like domain-containing proteins RELA/p65, RELB, NFKB1/p105, NFKB1/p50, REL and NFKB2/p52 and the heterodimeric p65-p50 complex appears to be most abundant one. The dimers bind at kappa-B sites in the DNA of their target genes and the individual dimers have distinct preferences for different kappa-B sites that they can bind with distinguishable affinity and specificity. Different dimer combinations act as transcriptional activators or repressors, respectively. NF-kappa-B is controlled by various mechanisms of post-translational modification and subcellular compartmentalization as well as by interactions with other cofactors or corepressors. NF-kappa-B complexes are held in the cytoplasm in an inactive state complexed with members of the NF-kappa-B inhibitor (I-kappa-B) family. In a conventional activation pathway, I-kappa-B is phosphorylated by I-kappa-B kinases (IKKs) in response to different activators, subsequently degraded thus liberating the active NF-kappa-B complex which translocates to the nucleus. NF-kappa-B heterodimeric p65-p50 and p65-c-Rel complexes are transcriptional activators. The NF-kappa-B p65-p65 complex appears to be involved in invasin-mediated activation of IL-8 expression. The inhibitory effect of I-kappa-B upon NF-kappa-B the cytoplasm is exerted primarily through the interaction with p65. p65 shows a weak DNA-binding site which could contribute directly to DNA binding in the NF-kappa-B complex. Associates with chromatin at the NF-kappa-B promoter region via association with DDX1. Essential for cytokine gene expression in T-cells (PubMed:15790681). [The UniProt Consortium]
| Schlagworte: | Anti-RELA, Anti-NFKB3, Anti-Transcription factor p65, Anti-Nuclear factor NF-kappa-B p65 subunit, Anti-Nuclear factor of kappa light polypeptide gene enhancer in B-cells 3, p65 Antibody NF-kB |
| Hersteller: | NSJ Bioreagents |
| Hersteller-Nr: | R30293 |
Eigenschaften
| Anwendung: | WB |
| Antikörper-Typ: | Polyclonal |
| Konjugat: | No |
| Wirt: | Rabbit |
| Spezies-Reaktivität: | human, mouse, rat |
| Immunogen: | An amino acid sequence from the N-terminus of human NF-kB p65 (AISQRIQTNNNPFQVP) was used as the immunogen for this p65 antibody (100% homologous in human and rat). |
| Format: | Purified |
Datenbank Information
| KEGG ID : | K04735 | Passende Produkte |
| UniProt ID : | Q04206 | Passende Produkte |
| Gene ID | GeneID 5970 | Passende Produkte |
Handhabung & Sicherheit
| Lagerung: | +4°C |
| Versand: | +4°C (International: +4°C) |
Achtung
Nur für Forschungszwecke und Laboruntersuchungen: Nicht für die Anwendung im oder am Menschen!
Nur für Forschungszwecke und Laboruntersuchungen: Nicht für die Anwendung im oder am Menschen!
Hier folgen Informationen zur Produktreferenz.
mehr
Hier kriegen Sie ein Zertifikat
Loggen Sie sich ein oder registrieren Sie sich, um Analysenzertifikate anzufordern.
Bewertungen lesen, schreiben und diskutieren... mehr
Kundenbewertungen für "Anti-p65 NF-kB"
Bewertung schreiben
Loggen Sie sich ein oder registrieren Sie sich, um eine Produktbewertung abzugeben.
Zuletzt angesehen



